
Industry Capabilities
Dedicated Site Solutions understands the challenges faced by the small/start-up pharmaceutical and biotechnology companies. Our team members have over 25 years of experience in the industry with successful track records of working on multiple approved products. We take on select projects and work within our means allowing us to offer personalized attention to your project needs and a quick start-up time.
What we offer for your projects: Medical Monitoring Our medical monitors come from diverse therapeutic
backgrounds and have the clinical and research expertise required to provide
medical oversight of your clinical trials.
Clinical Study Monitoring
Clinical trials are successful with efficient management and monitoring of clinical data. Dedicated Site Solutions monitoring team has an exceptional track record that ensures scientific excellence and data integrity across all sites. We engage a diverse group of specialized and people to perform all aspects of site management and monitoring.
We regionalize travel to limit costs to our client as efficiently as possible and have monitoring resources across the US states to accomplish this. On average our monitors have approximately 20 years of experience in monitoring. These stellar resources have experience in multiple oncology indications, including liquid and solid tumors and glioblastoma, chronic diseases, rare diseases, and device/diagnostics. Our monitors resources are well known and respected amongst many sites and with Key Opinion Leaders (KOLs) throughout multiple indications. These strong and respected relationships with a site can be one of the most valuable assets to a clinical study for any sponsor, ensuring adherence to data quality, subject safety and issue resolution throughout a clinical program.
Comprehensive monitoring plans are developed to address complexity and sponsor requirements, and often combine traditional monitoring visits with robust site management and remote monitoring activities.
Clinical Trial Monitoring activities include:
- Monitor visits qualification, initiation, routine, and
close-out
- Comprehensive monitor plans
- Expertise for feasibility or regulatory submissions
- 100% review of site records
- Timely completion of visit reports
- Quality review of monitor reports
- Source document verification as per monitor plans
- Rapid query resolution
- Site training and support
- Site management support and documentation
- Periodic remote EDC review
- Drug accountability
- Co-monitoring
- Rescue/SWAT monitoring
Project Management
Upfront planning results in fewer study delays and errors, assuring you receive high-quality study outcomes. Our clinical trial management philosophy supports all aspects of your early clinical trials cost effectively with clear roles and responsibilities.
Project Management Support:
- Develop the project plan and timeline
- Qualification, selection, and oversight of vendors
- Start-up activities and study initiation
- Oversee study conduct and clinical monitoring
- Site management support and documentation
- Manage data support teams from protocol development to clinical study report
- Maintain and report metric deliverables
- Provide for efficient project close
For additional information Email: info@dss-us.com
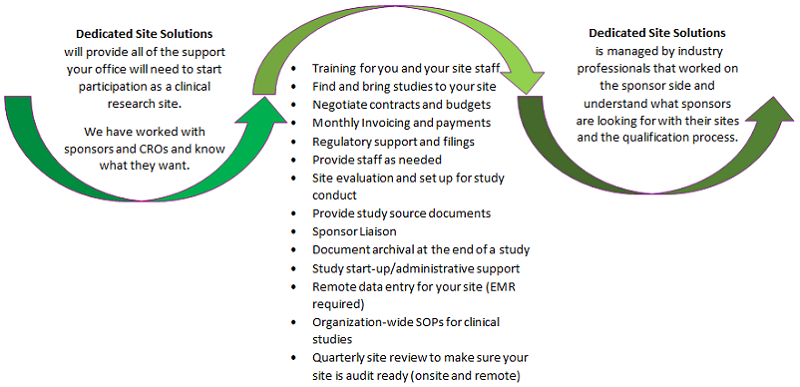
For Private Practice Physicians
Navigating the clinical trial process can seem overwhelming to a new site interested in entering clinical research
DSS is the resource for the private practice physician group to become recognized as a clinical practice site for participation as a clinical research Investigator site.
©2024 Dedicated Site Solutions, LLC - All Rights Reserved